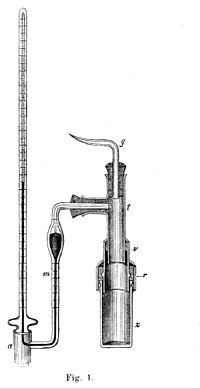
CHEM106S16 E3.pdf - CHEM 106 E3 Spring 2016 Name Constants and equations: 1 J = 1 kg∙m2∙s-2 Π=iCRT ΔT=iKfpm ΔT=iKbpm C=kP | Course Hero
![Welcome to Chem Zipper.com......: Decimolar solution of K4[Fe(CN)6] dissociate by 60% at 27°C. Determine osmotlc pressure in Nature/M2. Welcome to Chem Zipper.com......: Decimolar solution of K4[Fe(CN)6] dissociate by 60% at 27°C. Determine osmotlc pressure in Nature/M2.](https://lh3.googleusercontent.com/-Scet3fcHeWw/XsiaHk8xMpI/AAAAAAAAHm8/gwhzoNws9YYtSmNesnNkgkSgyki5Fg2aACLcBGAsYHQ/s1600/1590204946192896-0.png)
Welcome to Chem Zipper.com......: Decimolar solution of K4[Fe(CN)6] dissociate by 60% at 27°C. Determine osmotlc pressure in Nature/M2.

The avrage osmotic presure of human of blood is 7.8 bar at 27^(@)C. The concentration of an aqueous NaCI solution that could be used in the blood stream is :

Welcome to Chem Zipper.com......: 2 millimolar solution of sodium ferrocyanide is 60% dissociated at 27°C. osmotic pressure of the slotution is .
Q 17/30 At 12^° C the osmotic pressure of a urea solution is 500mm . The solution is diluted and the temperature is raised to 27^° C, when the osmotic pressure is

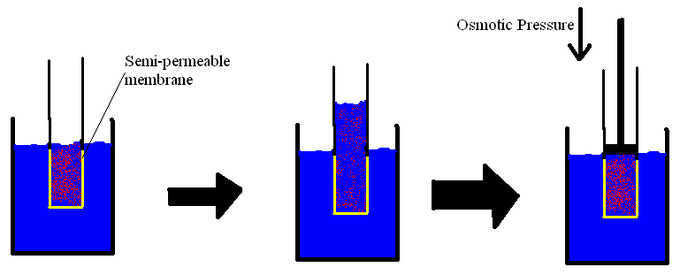
The osomotic pressure `pi` depends on the molar concentration of the solution `(pi=CRT)`. If two... - YouTube
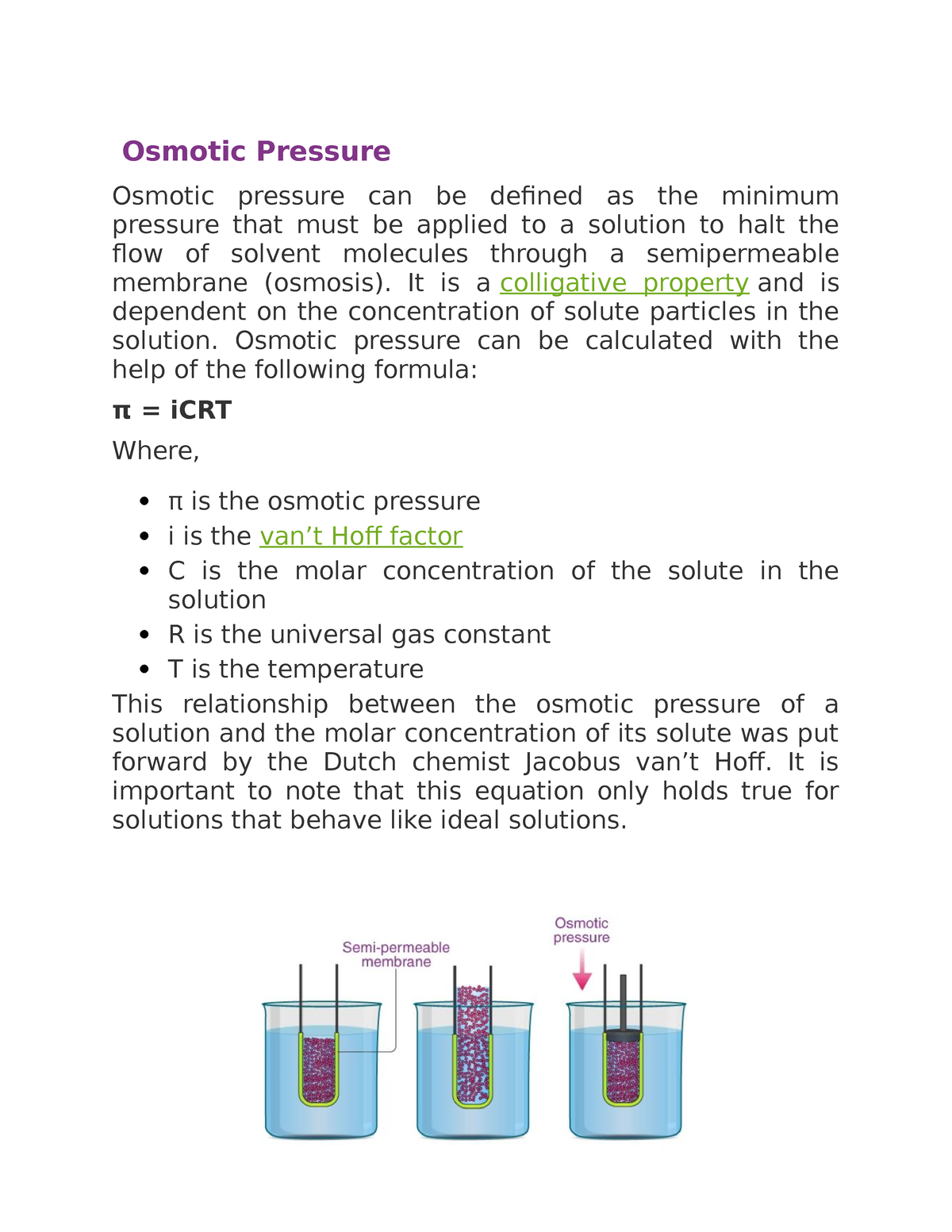
Osmotic pressure - Biochemistry - Osmotic Pressure Osmotic pressure can be defined as the minimum - Studocu
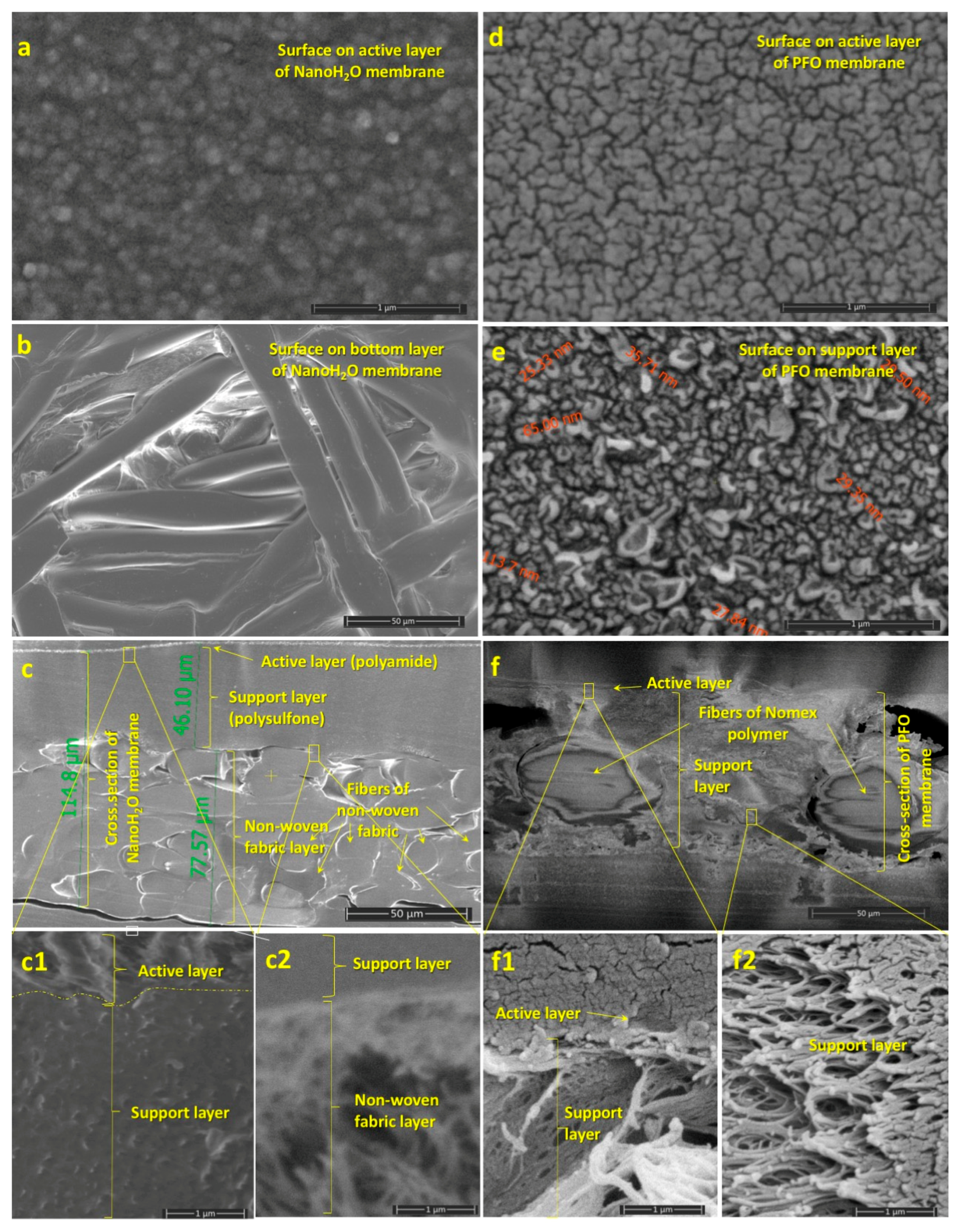
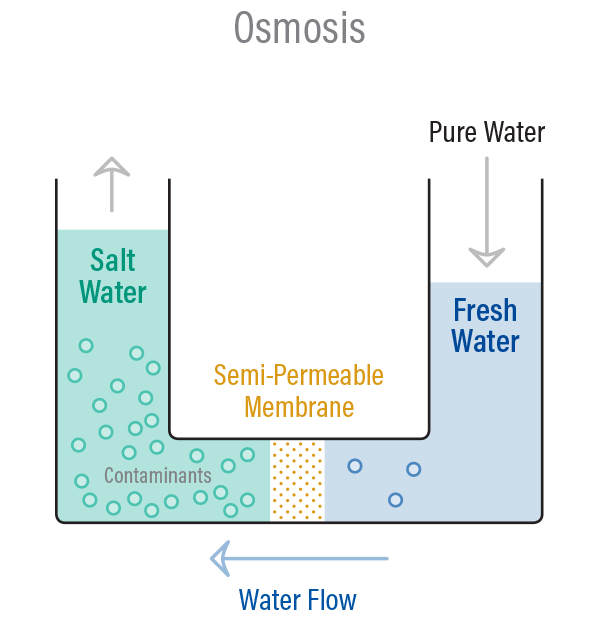
Membranes | Free Full-Text | The Need for Accurate Osmotic Pressure and Mass Transfer Resistances in Modeling Osmotically Driven Membrane Processes
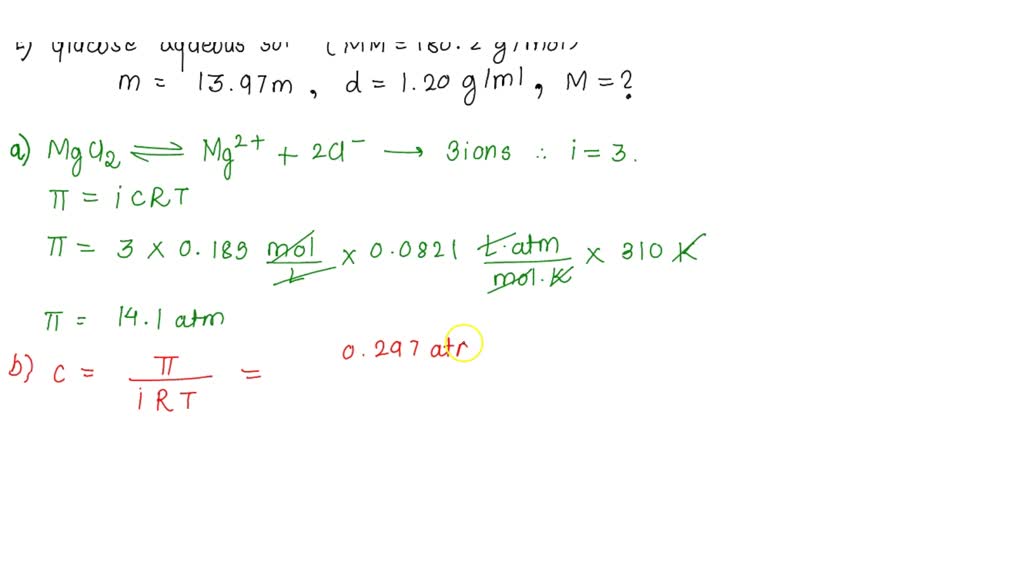
SOLVED: 20a.) What is the osmotic pressure, in atm, of a 0.185 M solution of MgCl₂ at 37.0 °C in atm? (assume complete dissociation). b.) What is the molarity of a solution