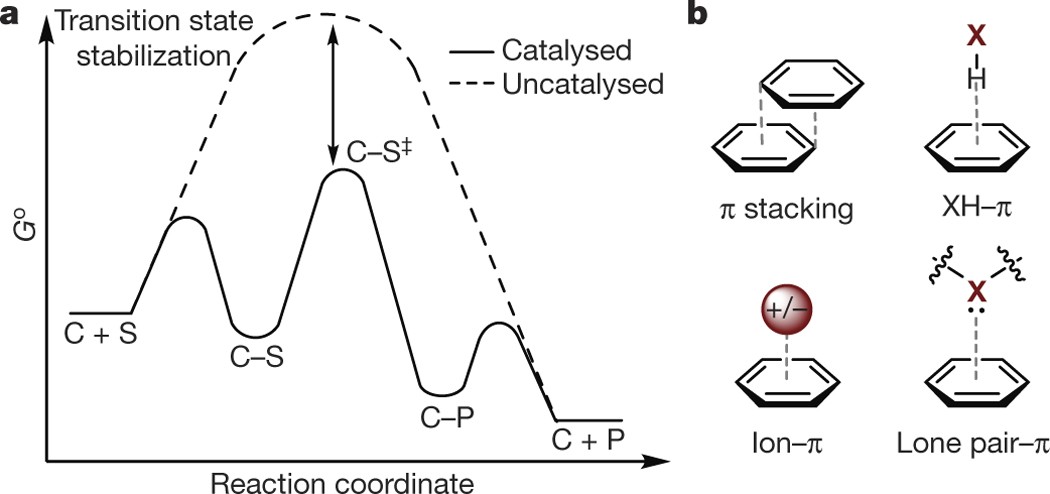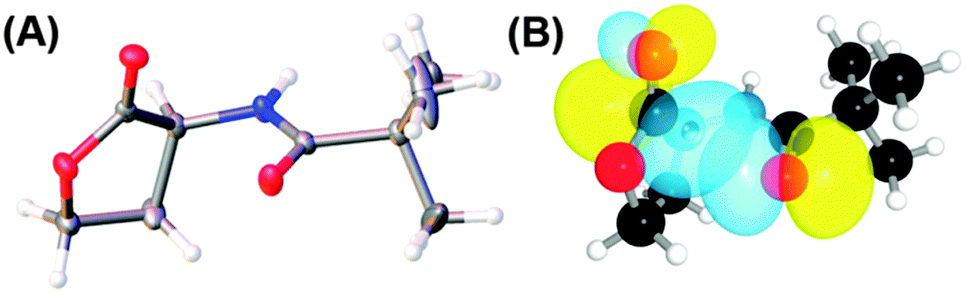
The n → π* interaction: a rapidly emerging non-covalent interaction - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C4CP05536E

Cation-π interaction and different geometries for S-H/π interactions.... | Download Scientific Diagram

Model interaction of NIK native ligand, apigenin, and luteolin. Dark... | Download Scientific Diagram
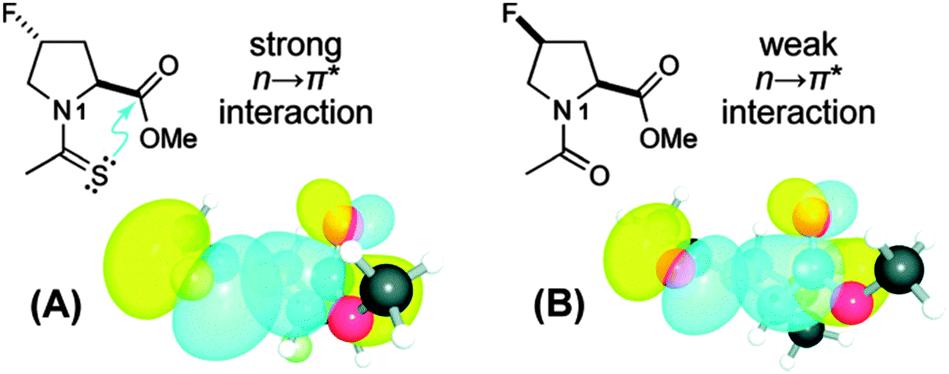
The n → π* interaction: a rapidly emerging non-covalent interaction - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C4CP05536E
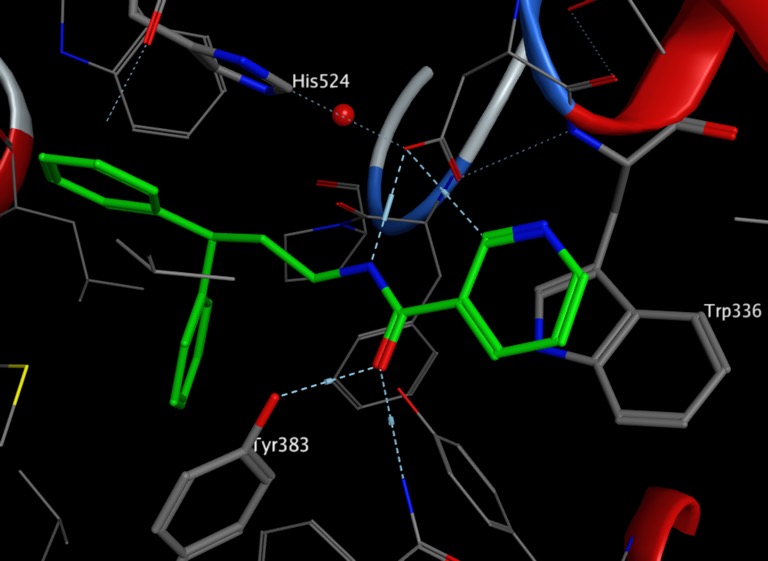
In silico analysis of binding interactions between GSK983 and human DHODH through docking and molecular dynamics

The interaction between methionine and two aromatic amino acids is an abundant and multifunctional motif in proteins - ScienceDirect

Noncovalent Interactions of π Systems with Sulfur: The Atomic Chameleon of Molecular Recognition - Motherwell - 2018 - Angewandte Chemie International Edition - Wiley Online Library

The Role of Tryptophan in π Interactions in Proteins: An Experimental Approach | Journal of the American Chemical Society
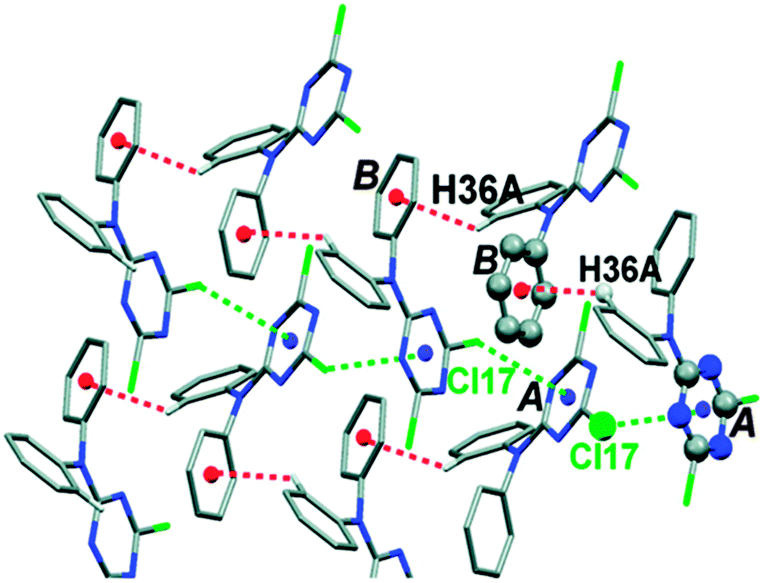
The n → π* interaction: a rapidly emerging non-covalent interaction - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C4CP05536E
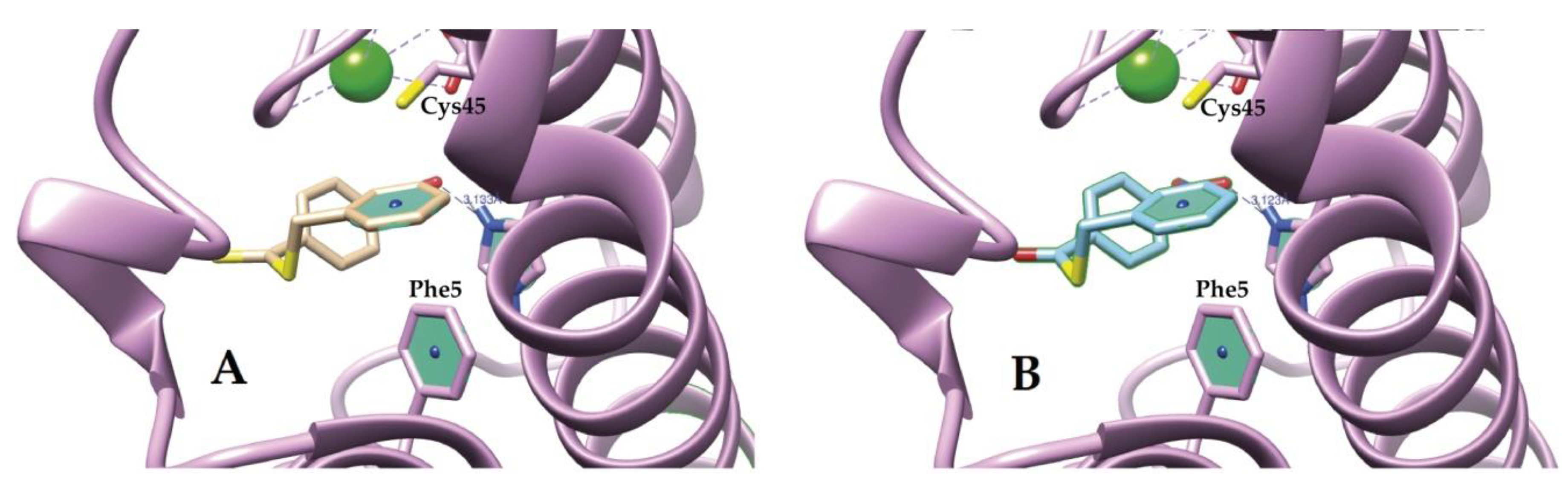
Molecules | Free Full-Text | Sulfur Compounds as Inhibitors of Enzymatic Activity of a Snake Venom Phospholipase A2: Benzyl 4-nitrobenzenecarbodithioate as a Case of Study
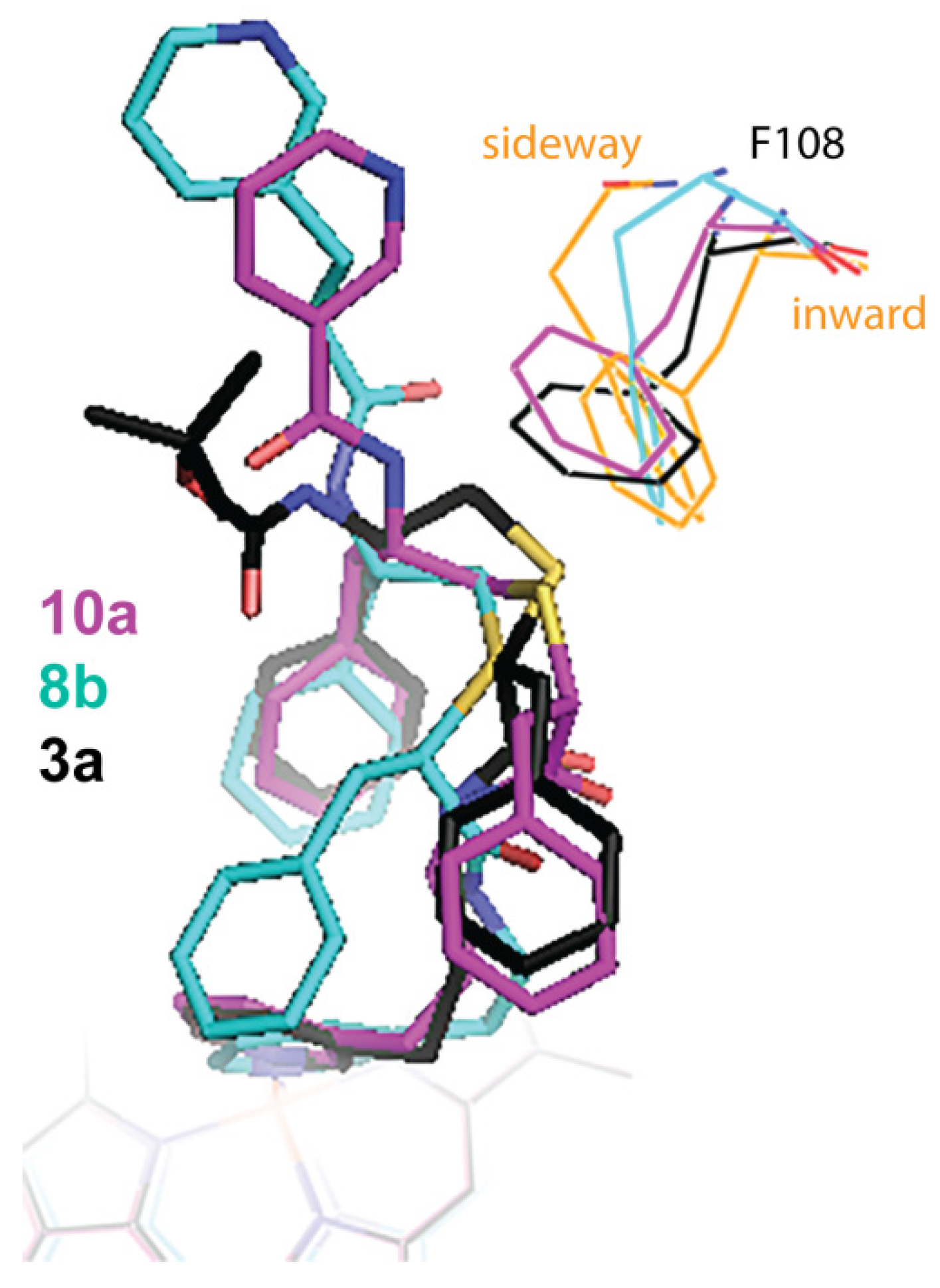
IJMS | Free Full-Text | Interaction of CYP3A4 with Rationally Designed Ritonavir Analogues: Impact of Steric Constraints Imposed on the Heme-Ligating Group and the End-Pyridine Attachment

Insights into Thiol–Aromatic Interactions: A Stereoelectronic Basis for S–H/π Interactions | Journal of the American Chemical Society

π-π stacking interactions: Non-negligible forces for stabilizing porous supramolecular frameworks | Science Advances
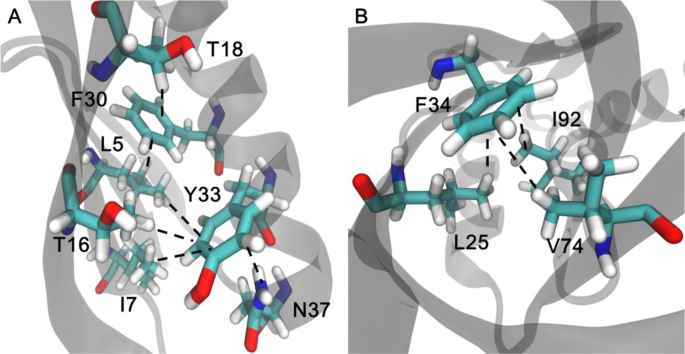
Dissecting C−H∙∙∙π and N−H∙∙∙π Interactions in Two Proteins Using a Combined Experimental and Computational Approach | Scientific Reports

Functionally Important Aromatic–Aromatic and Sulfur−π Interactions in the D2 Dopamine Receptor | Journal of the American Chemical Society

The interaction between sulfur and the π orbitals of the C=N links is far greater than anything which occurs with oxygen or nitrogen substituents.” “organic. - ppt download

Tailoring π-Conjugated Systems: From π-π Stacking to High-Rate-Performance Organic Cathodes - ScienceDirect